Seizures can impact people of all ages, from children to adults, and they often require…
Could endocannabinoid deficiency contribute to the development of neurodegenerative disorders
HOMEOSTATIC NETWORKING: THE ROLE OF PHYTOCHEMISTRY, THE ENDOCANNABINOID SYSTEM AND POTENTIAL FUTURE THERAPEUTICS
From an evolutionary perspective, what humans gain in intelligence is offset by the sacrifice lost in the unexpected change in our diet, where our gut biome now differs from what is evolutionarily expected of us. Our bodies have evolved to possess a certain gut microbial flora that is not as prevalent in modern society. Through technological progress that allows for the convenience of easily obtainable food sources with the advent of agricultural dependence some 500 generations ago, our divergence from the hunter-gatherer diet may have led our bodies astray – we no longer consume the diverse and prodigious phytochemicals as our paleolithic ancestors did.
Our phytochemical intake at present is estimated to be 8x less than modern humans (Easton SB. 2006. “The ancestral human diet: what it was and should it be a paradigm for contemporary nutrition.” Proc Nutr Soc 65(1):1-6). Could it be that all animals including humans evolved with an expectation that our gut microbial flora would contribute to an as yet to be understood homeostatic mechanism that helps keep us healthy without the development of chronic diseases with aging? Could it be that our diet supports directly and/or indirectly this as yet to be understood homeostatic network that is omnipresent within the body and brain? Could it be that the collective changes in our diet with modernity may be contributing to the malfunctioning of this theoretical homeostatic mechanism? Could this theoretical homeostatic network that we possess be the endocannabinoid system?
This will be an exploration of my understanding of diet and how it may relate to complex homeostatic networking within the brain and body. This exploration will help to introduce the concept of the endocannabinoid system and its role in maintaining cellular homeostasis. At the conclusion, I will demonstrate how modulation of the endocannabinoid system holds much promise in the future treatment of neurological disorders as well as other chronic medical conditions.
PHYTOCHEMISTRY & THE ROLE OF DIET IN HEALTH MAINTENANCE
At present, the average American BMI is 26 and the average serum total cholesterol is 294. The average BMI and total cholesterol of African hunter gatherers (most similar modern day human diet relative to paleolithic human diet) is 19 and 121 respectively. Americans and people who consume Western diets epidemiologically have higher rates of chronic disease relative to their African hunter-gatherer counterparts. Incidences of hypertension, diabetes, rheumatoid conditions, autoimmune conditions, inflammatory conditions, etc are much less in these populations who consume lesser calories and a more phytochemically diverse spectrum of foods.
While life is certainly “harder” for the African hunter-gatherer tribespeople of modern day, from a chronic health standpoint – these peoples may in fact be more adaptable in terms of their bodies’ abilities to adapt to stressors. With less incidences of chronic medical maladies in this cohort of people, perhaps their diets contribute to their body robust ability to stay “healthy” with age. Perhaps these groups of people who do not fancy a Western diet possess a robust homeostatic mechanism that allows them to be much more adaptable to stressors that would otherwise debilitate one with a more modern diet, less rich in phytochemical diversity. Adaptation, whatever the context, involves a progressive modification of some structure or structures. Perhaps, modern human divergence from a diverse phyotochemistry diet of yore has allowed for the promulgation of certain chronic conditions more commonly seen in modern day humans.
We know that a large number of dietary food components can exert effects on the human genome, either directly or indirectly, to modulate gene expression. We steep our genes daily in our dietary broth. Plants, in essence, shape humans – a well known example is the “shaping” of our cytochrome P450 genes. Phytochemistry is capable of altering the metabolism and potentially changing the biological fitness of humans as well as their domesticated animals, and even the obligate parasites of each species (Jackson FLC. “Secondary Compounds in Plants (Allelochemicals) as Promoters of Human Biological Variability. Annu Rev Anthropol. 1991; 20:506-546).
It is evident that the phytochemicals derived from a variety of plants can interact in additive and synergistic ways to modulate physiological function. The total dietary load of phytochemicals may have important implications for health. In prospective cohort studies, as well as case control studies and a few ecologic studies, reduced risk for coronary artery disease and for ischemic stroke has been linked to relatively high intakes of plant foods (McCarty, MF (2004). “Proposal for a dietary “phytochemical index”. Med Hypotheses 63, 813-7). Based on the wealth in data gleaned about the role of diet in health, it is a valid hypothesis to propose that the additive and synergistic effects of phytochemicals in fruit and vegetables are responsible for their potent antioxidant and anticancer activities. In fact, human epidemiologic data indicate that susceptibility to adult-onset chronic disease is influenced by persistent adaptations to prenatal and early postnatal nutrition (Waterland RA & Jirtle RL. 2003. “Early Exposure to Phytochemistry” Mol Cell Biol. 23(15): 5293-5300).
Clinicians, myself included, have long neglected the role of diet in the maintenance of health. As I age, I am becoming much more cognizant of the importance diet plays in regulating my body’s immune system and overall function. Simple changes such as limiting processed grains (breads) has helped me have less cravings and improved my energy levels. Using more herbs and eating more vegetables has helped me cut my cholesterol down naturally, without the usage of antihypertensive medications. As a doctor, I am starting to realize that the nature of the disorders I treat (in neurology) are much more complicated than the simplistic approach the drugs I use to treat some of these disorders require, drugs that only target one specific receptor – aka “clean drugs”.
Complicated disorders cannot simply fit into simplistic models – made just so that people can understand them better. The genetic component of all complex traits, such as hypertension, arthritis, and cognitive function, is influenced by a broad range of effects spread across many single nucleotide polymorphisms in many genes (Cooper RS. 2003. “Disease as Multifactorial” Ann Intern Med 139:437-40). Thinking of chronic diseases as being related to multifactorial etiologies makes much more sense than thinking from the simplistic paradigm that diseases are caused by only one specific and isolated pathophysiological process. Complicated neurological disorders must be viewed as a whole – a systems problem, a network biological problem. Network biology theory predicts that modulating multiple nodes simultaneously is often required for modifying phenotypes. When thinking about pathophysiology affecting networks, the usual mathematical techniques of linear approximation-linear regression, normal coordinates, mean field approaches, and the like – make little progress in the analysis of complex adaptive systems. This seems to be why simplistic approaches to “solve” neurodegenerative disorders like Alzheimers disease (through the clearance of beta-amyloid plaques) have only led to failures. Perhaps, taking this “big picture” approach to neurological disorders is the paradigm that scientists must now think within as it will then help to understand that complicated network problems may actually be related to more simplistic overall dysfunction of a common connected system. It becomes clearer now to see health dysfunction as being related to dysregulation of homeostatic mechanisms and seemingly unrelated disorders now seem related when you think of these disorders from this perspective.
The allowance of the body to “heal itself” has been the antithesis of what allopathic medicine is. Allopathic medicine teaches us that there is a pharmaceutical and/or surgical fix to a problem and therapeutics have always developed from this basic “rule.” Now the concept of “restoring cellular homeostasis” is allowing for more insight into thinking about diseases from the systems biology standpoint and new pharmaceutical approaches can now be made to enhance the body’s natural ability to heal itself.
Network pharmacology allows for the recognition, informed by systems biology, that drugs for many disease states may require multiple activities to be efficacious, together with the observed promiscuity of old, small-molecule drugs, points strongly at the potential efficacy of drugs that have multiple targets (aka “dirty drugs”) and medicinal plants as effective therapeutics. Compounds that selectively act on two or more targets of interest in theory should be more efficacious than single-target agents when through of from the network biology perspective. Pharmacological strategies with multiple targets might have a better change of affecting the complex equilibrium of whole cellular networks than drugs that act on a single target.
Diets rich with phytochemical diversity may serve to maintain gut microbiota to be within an evolutionarily expected optimal balance that in turn, primes the body to maintain homeostasis through the proper functioning of the body’s endocannabinoid system. With a deficient endocannabinoid system, homeostatic mechanisms are impaired and there is an increased risk for the development of chronic health conditions – including many neurological disorders like dementia, epilepsy, etc. It is with this understanding that I hypothesize that all humans have an underlying “endocannabinoid tone” that is a reflection of levels of anandamide (AEA) and 2-AG (endogenous cannabinoids), their production, metabolism and the relative abundance and state of cannabinoid receptors. Based on this hypothesis, I theorize that in certain conditions, whether congenital or acquired, endocannabinoid tone becomes deficient allowing for the production of pathophysiological syndromes.
ENDOCANNABINOID PHYSIOLOGY:
In order to understand the new systems/network biological approach of drug development for enhancing CNS and body homeostasis, it is very important to start understanding the endocannabinoid system. Know that many health conditions are influenced by cannabinoids – examples being: ADD/ADHD, Alzheimer’s, ALS, asthma, bipolar, cancer, chronic fatigue, crohns, diabetes, epilepsy, migraine, multiple sclerosis, nausea, neuropathy, rheumatoid arthritis, stroke, Tourette, spasms, etc. Why does one herb, cannabis, seem to help so many different conditions? For that matter, why does one experimental drug, Anavex 2-73, seemingly help with so many different conditions? The answer simply may be the endocannabinoid system is enhanced. Endocannabinoid synthesis is an adaptive response to cellular stress, aimed at reestablishing cellular homeostasis.
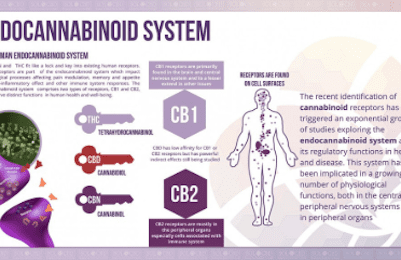
The two well-known cannabinoid receptors, CB1 and CB2, exist within the human body. CB1 receptors are mostly located in the CNS, as well as heart, GI tract, liver, connective tissue, adipose tissue, testes/uterus. CB2 receptors are mostly located in immune-related organs: monocytes, macrophages, B-cells, T-cells, liver, spleen, tonsils, enteric nervous system and also the CNS.
CB1 receptors are the most common G-protein coupled receptors in the brain with highest densities within the hippocampus, cerebral cortex, cerebellum, amygdala and basal ganglia but are virtually absent in the brainstem (hence no lethal effect of overdose – no major cardiorespiratory effects with cannabis usage). CB1 receptor activity effects short-term memory, cognition, mood and emotion, motor function and nociception. CB2 receptors seem to play more of an immunological role and “healing” role after injury and may be important in having an anti-inflammatory role as well. At the synapse, cannabinoid activity enhances retrograde signaling – i.e. stimulates the presynaptic nerve terminal to inhibit release of excitatory neurotransmitters to the synaptic cleft. This is known as depolarization-induced suppression of excitation – cannabinoids like AEA essentially enhances the closing of presynaptic calcium channels to stop vesicle release of excitatory neurotransmitter glutamate into the synaptic cleft. Cannabinoids may also contributed to depolarization induced suppression of inhibition – involving similar mechanisms.
Cannabinoid receptors evolved some 600 million years ago – insects are the only organisms that do not possess cannabinoid receptors in this day/age among all animals. Human CB receptors are similar to CB receptors in simple and evolutionarily old organisms – such as the sea squirt. Just this fact alone suggests that cannabinoid receptors play a vital role in all organisms except insects. Cannabinoid receptors can activate different G protein subtypes that affect ion channels or that inhibit or stimulate adenylate cyclase.
Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are two known endogenous cannabinoid ligands that are considered to be retrograde messengers in the nervous system and are autocrine/paracrine mediators elsewhere. These endogenous cannabinoids are synthesized on demand locally from cell membrane precursors, released immediately and then rapidly metabolized locally. There are many other endogenous cannabinoids as well – all playing a role that is yet to be elucidated – within the endocannabinoid system. Overall, the agonist trafficking and the overlap of such trafficking with other systems other the endocannabinoid system can explain the complex effects cannabinoids have on brain and body chemistry.
The endocannabinoid system is involved in the function and regulation of the nervous system, connective tissues, immune system, neoplastic processes, embryology, digestive system and hunger/feeding. Cannabinoids help to modulate neural plasticity and are heavily involved in learning. Cannabinoids are involved in neural protection – AEA and 2AG are endogenous neuroprotective agents produced by the nervous system upon both chemical and mechanical trauma (Mechoulam, 2002). THC-A, CBD, AEA, 2AG and HC-210 all decrease glutamate excitotoxicity and reduce seizure activity as well as limit infarct size post-stroke (Baker, 2003) (if I have a stoke in progress, the protocol for my wife is call 911, give me an aspirin and give me a joint or THC to increase vasodilation and to help minimize the penumbra zone surrounding the stroke cavity). Cannabinoids have been shown to be effective at reducing and preventing perinatal brain injury (Fernandez-Lopez et al, 2013). In fact, the US government owns a patent attesting to cannabinoids as antioxidants and neuroprotectants (see US patent No 6630507 B1 – 10/7/2003). CB1 receptors are involved in regulating autonomic tone: in the sympathetic nervous system it inhibits norepinephrine release, dampens sympathetically mediated pain, modulates the hypothalamic-pituitary-adrenal axis and hypothalamic-locus coeruleus-norepinephrine axis and in the parasympathetic nervous system, it reduces elevated activity providing the antiemetic effects of cannabinoids. CB1 activity has effects on heart rate and cardiac contractility as well as vasodialatory activity and plays a protective role in myocardial ischemia as suggested in rodent studies (Pacher, 2006).
Preclinical models show endocannabinoid system activation causes antinociceptive effects in acute pain, persistant inflammatory pain and neuropathic pain. CB1 signaling at the site of nerve injury is the first line of defense against pain – it decreases the release of activators and sensitizers around the site of tissue injury and opens K+ channels in the nociceptor cell membrane so the nerve becomes hyperpolarized and less likely to fire. CB2 signaling decreases release of activators and sensitizers from neighboring mast cells and macrophages (Walker, 2005). Cannabinoid receptors and opioid receptors are both present in pain signaling regions of the brain and spinal cord – opioid and cannabinoid signaling pathways interact with each other and administering cannabinoids with opioids results in a greater than additive antinociceptive (anti-pain) effect (Cichewicz, 2004).
Endocannabinoid activity has been demonstrated in bone osteoblasts/osteoclasts – involved in bone healing following injury and maintenance of bone health. There is endocannabinoid activity in connective tissue – fibroblasts, myofibroblasts, chondrocytes, synoviocytes all express CB1, CB2 and endocannabinoid metabolizing enzymes. In fact CB1 levels are upregulated after exposure to inflammatory cytokines and equiaxial stretching of fibroblasts and cannabinoids modulate fascial remodeling via fibroblast focal adhesions (McPartland, 2008). Cannabinoids dampen the inflammatory component of atherosclerosis in animal studies via CB2 receptors expressed by macrophages within atherosclerotic plaques.
Endocannabinoid activity is heavily involved in the mediation of the immune system. Cannabinoids have been shown to inhibit tumor grown in multiple cell line xenografts – antineoplastic properties may occur for multifactorial reasons. Cannabinoid activity is involved in embryogenesis and suckling activity in newborns. Endocannabinoids within the digestive system help regulate the enteric nervous system, inhibit gastric acid secretion.
Cannabinoid receptor polymorphisms have been associated with certain neuropsychiatric conditions: addictions, schizophrenia subtypes for example. CB receptor polymorphisms may be related to other numerous and different neurological and non-neurological conditions like central obesity, hyperlipidemia, cyclic vomiting syndrome (may be a manifestation of endocannabinoid system overactivity).
The endocannabinoid system is widely distributed throughout the body and is widely misunderstood. We do not learn much about it in medical school and we do not know its essential function though – numerous studies point to its role in helping to regulate cellular homeostasis. Modulating the endocannabinoid system may have therapeutic potential in almost all disease affecting humans, including obesity/metabolic syndrome, diabetes and diabetic complications, pain, neurodegenerative diseases, inflammatory disorders, cardiovascular disorders, hepatic disorders, gastrointestinal and skin diseases; psychiatric disorders, cachexia; cancer and chemotherapy-induced nausea/vomiting amongst others (Pacher, et. al “Modulating the endocannabinoid system in human health and disease-successes and failures”. FEBS Journal 280.9 (2013): 1918-1943).
There needs to be a lot more research on cannabinoids as medical therapeutics. We are now at the beginning stage of a revolution in neurological therapeutic agents in regards to the use of cannabinoids. In New Jersey, I am excited about the prospects of being able to do clinical research on the use of cannabinoids for treatment of neurological conditions. We need our federal government to be more supportive by funding more clinical research in this regard.



